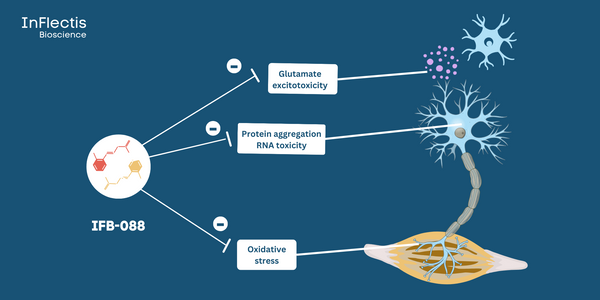
Nantes, France -- January 3, 2024 -- InFlectis BioScience, a pioneer in developing first-in-class therapies for neuromuscular diseases, announced that it has been awarded a $943,000 grant to support recruitment and to investigate biomarkers in its Phase 2 clinical trial in patients with bulbar-onset ALS (NCT05508074). The trial is investigating IFB-088 (INN: icerguastat), a multifunctional drug that modulates the PPP1R15A/PP1c phosphatase complex and inhibits NR2B-containing NMDAR. The potential of IFB-088 lies in its ability to target three major ALS cellular pathophysiological mechanisms to manage disease progression: protein aggregation, oxidative stress, and glutamate excitotoxicity.
The purpose of biomarker identification is to ascertain the existence of additional candidates that may be linked to IFB-088's effects on protein aggregation, oxidative stress, and glutamate excitotoxicity. These biomarkers could play a crucial role in assessing IFB-088's efficacy in larger clinical trials and potentially aid in its regulatory approval.
The randomized, double-blind, placebo-controlled phase 2 study will enroll 50 patients with bulbar-onset ALS and compare the effects of IFB-088 plus riluzole (current standard of care in Europe) and placebo plus riluzole for a six-month treatment period. InFlectis expects patient recruitment to be completed in France and Italy by the end of January 2024, with results reported as early as the end of 2024.
InFlectis's COO, Pierre Miniou, Ph.D., explained, “This grant and the support from the ALS Association are a testament to the potential of IFB-088. We are committed to exploring all avenues to bring effective solutions to those suffering from ALS and other rare neuromuscular diseases."
The ALS Association, the largest philanthropic funder of ALS research in the world, supports early-stage clinical development of promising new treatments through its Clinical Trial Awards. Paul Larkin, Ph.D., Director of Research at the ALS Association, stated, “We need more treatments as urgently as possible to help make ALS a livable disease until we can cure it. IFB-088 takes a novel approach, acting on several pathophysiological mechanisms, and we eagerly await the results of this and any future studies."
“This trial first investigates patients suffering from bulbar-onset ALS, which is recognized as the most devastating presentation of the disease. It utilizes a molecule targeting key pathophysiological mechanisms, and we are confident that it will confirm the positive results obtained from a previous study we published in Brain two years ago,” commented Prof. Giuseppe Lauria, M.D., Scientific Director of the IRCCS Foundation "Carlo Besta" Neurological Institute in Milan, Italy, that will be collaborating with InFlectis.