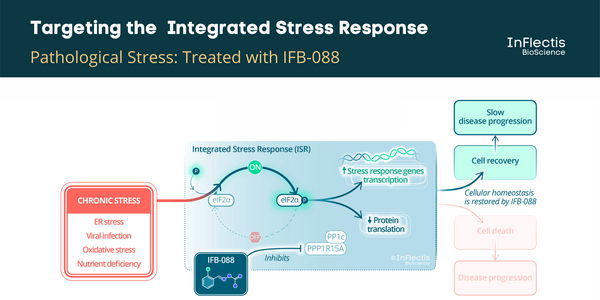
Nantes, France -- March 28, 2022 -- InFlectis BioScience received Orphan-Drug Designation from the U.S. Food and Drug Administration (FDA) for its investigational treatment for Amyotrophic Lateral Sclerosis (ALS), IFB-088. In preclinical studies, IFB-088 has been shown to prolong the protective effect of the Integrated Stress Response, giving cells additional time to repair or eliminate mis-folded proteins that can result in diseases such as ALS. The Company plans to initiate a Phase 2 trial in Europe (France and Italy) this summer. Orphan drug designation provides for tax credits for qualified clinical trials and extended market exclusivity for up to seven years as a way to encourage companies to develop treatments for rare conditions.
“The granting of ODD reflects the need for new treatment options with new mechanisms of action for patients diagnosed with ALS. This is an important milestone for the company and a significant step forward in our US and European clinical development and regulatory strategy for IFB-088,” said Béatrice Lejeune, Ind. Pharm., Chief Regulatory Officer of InFlectis. “Phase 2 clinical data from an analog to IFB-088 reported last year demonstrates the potential of an ISR modulator to slow the progression of ALS, in particular in bulbar-onset ALS patients.
“We are now preparing the setup of clinical operations and planning to enroll the first patient this summer,” explained Philippe Guédat, PhD, Founder, President, Chief Executive Officer of InFlectis. “In our development plan we expect to initiate larger studies in the U.S. and worldwide for the whole ALS population.”
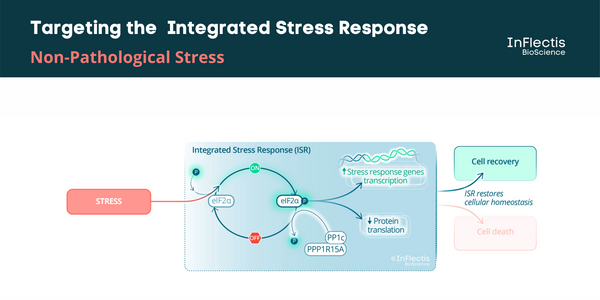
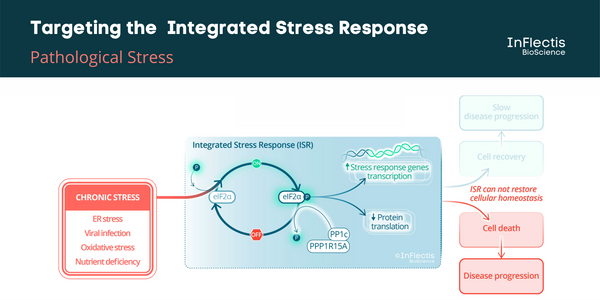
ISR Pathway and ALS
It has been demonstrated that targeting the unfolded protein response (UPR) pathway, a part of ISR, in particular the PPP1R15A/PP1c phosphatase complex, could be beneficial to ALS patients. A PPP1R15A/PP1c phosphatase complex inhibitor, guanabenz, has been investigated in an academic randomized Phase 2 study in ALS patients. It showed encouraging results, especially in patients with bulbar-onset ALS. However, the incidence of hypotension in this study discouraged further development in this indication. A similar efficacy is expected for IFB-088 without hypotensive effects.
Amyotrophic Lateral Sclerosis
ALS is a neurological disease characterized by degeneration of upper and lower motoneurons leading to muscle weakness and subsequent paralysis. Currently, there is only one drug available in Europe, riluzole. ALS can be divided clinically into 2 forms depending on the type of first symptoms, namely spinal- and bulbar-onset diseases, the latter being characterized by difficulties in swallowing, chewing, and speaking, a more rapid evolution and a poorer prognosis.