Nantes, France, January 31, 2024 -- InFlectis BioScience, a pioneer in developing first-in-class therapies for neuromuscular diseases, announced that it has completed enrollment in its Phase 2 clinical trial of IFB-088 ((INN: icerguastat), a novel investigational treatment of Amyotrophic Lateral Sclerosis (ALS).
“This trial reflects our commitment to bring a novel molecule with a unique mode of action to ALS patients, starting with patients suffering from bulbar-onset ALS, the most aggressive form of the disease,” said Anne Visbecq, Chief Medical Officer, InFlectis BioScience. “We dosed the first patient in December 2022 and randomized the last patient on January 30, 2024. Following the six-month treatment period, we look forward to reporting our initial findings as early as the end of this year. These results will determine the timing and scope of a potential global registration study in the overall ALS population.”.
The randomized, double-blind, placebo-controlled phase 2 trial enrolled 50 patients with bulbar-onset ALS in investigational sites in France and Italy. Bulbar-onset ALS accounts for approximately 25% to 30% of patients diagnosed with ALS and it is the most aggressive form of the disease with a shorter life expectancy compared to spinal-onset ALS. It is characterized by difficulties in chewing, swallowing or speaking as initial signs and symptoms. The trial is comparing IFB-088 plus riluzole (current standard of care in Europe) with a placebo plus riluzole for a six-month treatment period. The Principal Investigators are Prof. Shahram Attarian from Hospital La Timone in Marseille, France and Prof. Giuseppe Lauria from the Istituto Neurologico Carlo Besta, Fondazione IRCCS, in Milan Italy.
The development of IFB-088 and the phase 2 trial in bulbar ALS patients were possible thanks to the financial support of:
- The ALS Association, the largest philanthropic funder of ALS research in the world (Press Release, DOI: 24-CTA-713);
- AFM-Téléthon, a French patient organization fighting rare neuromuscular diseases (Press Release);
- bpifrance, the French public bank of investment (PIA - Investments for the Future Programme).
About IFB-088 (INN: icerguastat)
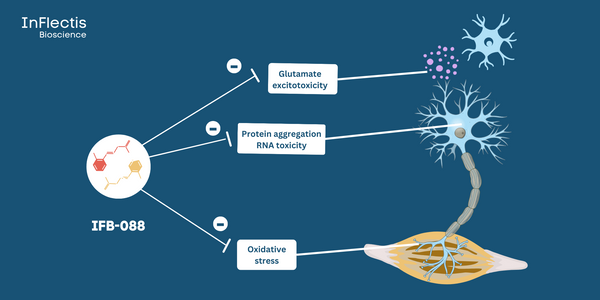
IFB-088 is a first-in-class orally available small molecule drug candidate with a validated mechanism of action and a promising pharmacokinetic profile capable of crossing the blood brain barrier to target the central and peripheral nervous system. IFB-088 is a multifunctional drug that modulates the PPP1R15A/PP1c phosphatase complex and inhibits NR2B-containing NMDAR. The potential of IFB-088 lies in its ability to target three major ALS cellular pathophysiological mechanisms to manage disease progression: protein aggregation, oxidative stress, and glutamate excitotoxicity.